Background
Olverembatinib (HQP1351) is a novel third-generation tyrosine kinase inhibitor (TKI) that has shown remarkable efficacy and safety in chronic myeloid leukemia (CML). Still, few reports are available on BCR::ABL1-positive acute lymphoblastic leukemia (BCR::ABL1+ ALL). We first reported the early encouraging outcome of olverembatinib in BCR::ABL1+ ALL patients (pts) with T315I mutation or disease progression last year. Here, we enlarged the sample size and included a case of CML in lymphoid blast phase (CML-LBP) who also benefited from olverembatinib to a certain extent.
Methods
In this observational study, we retrospectively enrolled BCR::ABL1+ ALL or CML-LBP pts with T315I mutation, disease progression, or intolerant to previous TKIs in our institution from Dec. 2021 to May 2023. All pts received olverembatinib monotherapy (40mg qod) or combination therapy. Efficacy was assessed by CR rate, MRD negative (MRD <10 -4) rate by multiparameter flow cytometry (MFC), CMR (BCR-ABL1 transcript<10 -5) rate by RT-qPCR, as well as the survival data. PFS and OS were both calculated from the start of olverembatinib. Adverse events (AEs) were assessed and graded according to CTCAE v5.0.
Results
Totally, 30 pts with BCR::ABL1+ ALL (n=29) or CML-LBP (n=1) were analyzed. Among 29 BCR::ABL1+ ALL pts (median age of 60 years [range, 23-76]), 14 were in CR with molecular R/R disease, including 1 with MRD only detected by RT-qPCR. While the other 15 had hematological R/R disease, including 4 with central nervous system leukemia. T315I mutation was detected in 23 pts, including 8 with additional mutations. The baseline characteristics are detailed in Table 1.
As for the 23-year-old female pt with CML-LBP, dasatinib was switched to olverembatinib when T315I, E255K, and E255V mutations were detected. T315I and E255V were eliminated rapidly after 1-month treatment of olverembatinib, while E255K still existed. At present, the patient maintained in CR, and blinatumomab will be used for MRD clearance before allo-HSCT. She was only included in the safety analysis.
Among 14 pts with molecular R/R disease, 61.5% and 50% achieved MRD flow negativity and CMR with a median time of 1.3 months (range, 0.8-4.4) and 1.2 months (range, 0.9-4.4), respectively. Among 15 pts with hematological R/R disease, 80%, 53.3%, and 46.7% achieved CR/CRi, MRD flow negativity, and CMR, with a median time of 1 month (range, 0.2-2.5), 1 month (range, 0.2-3.3) and 2.1 months (range, 0.7-4.6), respectively. Totally, 8 pts underwent allo-HSCT with a median age of 48.5 years (range, 23-60), of which 6 pts achieved CMR before allo-HSCT, and all attained persistent CMR after allo-HSCT.
By June 15, 2023, with a median follow-up time of 9.1 months (range, 1.7-16) in BCR::ABL1+ ALL pts, the median PFS was 7.8 months, and the median OS was not reached. For molecular R/R pts, the median PFS and OS were not reached, and for hematological R/R pts, the median PFS and OS were 6.5 and 8.6 months. MRD flow negativity or CMR responders had significantly better PFS and OS (PFS: p<0.001, p<0.001; OS: p<0.001, p<0.001), and bridging allo-HSCT after olverembatinib-based therapy showed a significant survival superiority (PFS: p=0.001; OS: p=0.017).
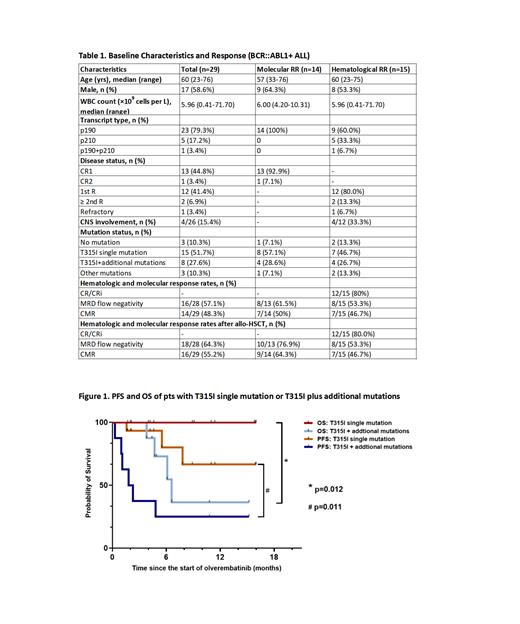
Among the 23 pts with T315I mutation, the MRD flow negativity rate in pts with T315I single mutation was significantly higher than those with additional mutations (p=0.008), and the CMR rate tended to be better (p=0.089). Meanwhile, pts with T315I single mutation showed significant improvements in PFS and OS (Figure 1).
As for safety analysis, the olverembatinib-based therapy was well-tolerated. Most non-hematological AEs were G1-2, and most G3-4 AEs were hematological, possibly related to chemotherapy. The incidence of G3-4 cardiovascular AEs was 6.7%. Five elderly pts (median age of 72 years [range, 63-76]) had temporary treatment suspension or dose reduction. No permanent discontinuation or death related to olverembatinib was observed.
Conclusion
This study further confirmed the efficacy of olverembatinib-based therapy in Chinese adults BCR::ABL1+ ALL pts with T315I mutation or R/R disease, especially in those with T315I single mutation. In addition, bridging allo-HSCT after deeper molecular remission could further improve survival. The safety profiles were manageable, but there is still a need to explore the optimal dose in elderly pts.
OffLabel Disclosure:
No relevant conflicts of interest to declare.
Olverembatinib is a novel 3rd-generation TKI used for CML and BCR::ABL1+ ALL

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal